Quality
At SLINDO, quality is the responsibility of everyone involved in the product life cycle, which is why there are several steps we take to meet our quality standards.
The first step is the sourcing of quality assured products from FDA registered manufacturers with FDA registered NDCs (or) EU GMP certified manufacturers. SLINDO is present in a major pharmaceutical hub, sourcing and securing high quality products that conform to the latest editions of USP, BP, EP and other compendial pharmacopeias.
Compliance
We check for non-compliance/notices of concern/warning letters issued against the supplier by verifying against the relevant websites. Searches against each new supplier will go back upto 5 years to ensure full compliance standards.

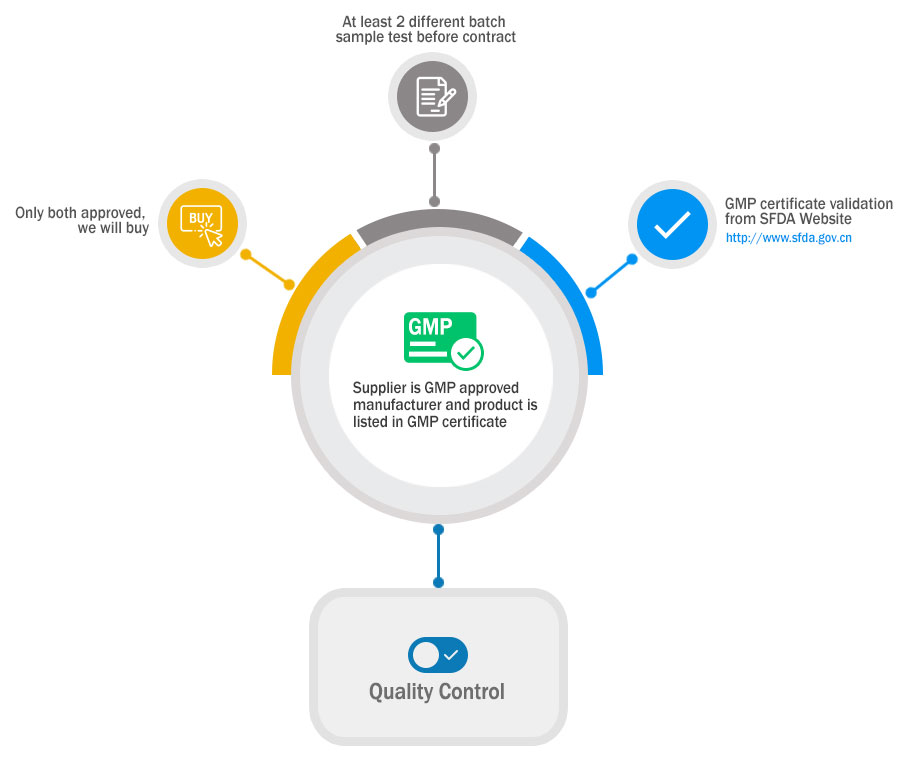
Dedicated to the Highest Quality of the Product
We adhere to the (GMP) codes manufacturing practice. We provide material safety data sheets(MSDS) and (C of A’s) certificates of analysis with all our supplied material. Our products are bought by trusted partners and we are always searching the world for the best products and price knowing quality and purity of the product is our main goal.

US FDA Compliance
SLINDO deals only with US FDA compliant manufacturing partners. We are dedicated to supplying active pharmaceutical ingredients and fine chemicals in both Canada and the USA. We specialise in sourcing and supply of premium compounding chemicals for pharmacies and health professionals who require the highest quality materials for their compounding needs.

Health Canada Compliance
Our Products are sourced, manufactured, and controlled according to the highest quality assurance standards of Health Canada. For over 8 years, we have been driving excellence in chemicals, oils and active pharmaceutical ingredients. All our products are sourced from cGMP and Health Canada registered manufacturers which confirm to current grades of USP and other compendia standards.